
Located above the mesosphere, the thermosphere is a very hot layer of the atmosphere.
- Subject:
- Science
- Provider:
- Utah Education Network
- Author:
- Visual Learning Company
- Date Added:
- 02/28/2010

Located above the mesosphere, the thermosphere is a very hot layer of the atmosphere.
The tropopause separates the troposphere from the stratosphere.
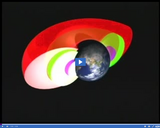
The belt of charged particles surrounding the Earth, referred to as the Van Allen Radiation Belt, is located in the magnetosphere.
A Powerpoint guided lesson about atmospheres (April Mitchell)

In this feature, adapted from Interactive NOVA: "Earth," students explore the relationship between oxygen concentration and the well-being of various organisms by simulating a change in oxygen levels and observing what happens.

In this interactive activity from ChemThink, take a closer look at atomic structure, properties, and behaviors.
Explore the interactions between various combinations of two atoms. Turn on the force arrows to see either the total force acting on the atoms or the individual attractive and repulsive forces. Try the "Adjustable Attraction" atom to see how changing the parameters affects the interaction.
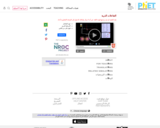
Explore the interactions between various combinations of two atoms. Turn on the force arrows to see either the total force acting on the atoms or the individual attractive and repulsive forces. Try the "Adjustable Attraction" atom to see how changing the parameters affects the interaction.

Students will use what they know about atoms & molecules to decide whether an example is an atom or a molecule.
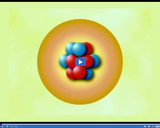
The number of protons of an element is constant, but the number of neutrons can change. Isotopes are atoms of the same element with different numbers of neutrons.

Carbon can exist as carbon 12 or carbon 14.

Compounds are formed by joining atoms of two or more elements. In this animation an ionic bond holds atoms together.

Atoms of the same element are exactly alike. This animation depicts a copper atom.

Introduce students to atoms with an engaging Google Slides and Cornell Notes activity. Image: jayofboy - https://www.freeimages.com/photo/atom-1307057
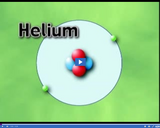
The four atoms depicted here have a different atomic number, which refers to the number of protons in the nucleus. The atomic number of helium is 2, aluminum is 13, hydrogen is 1, and oxygen is 8.

Matter, such as this ice cream, is made up of many atoms.

Replicates the experiments J.J. Thomson conducted to develop his model of an atom. Light is produced when an electric current is passed through a tube. When a magnetic charge is introduced, the rays are deflected.
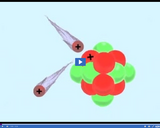
Like charges repel one another.

The mass number of an atom is the sum of its protons and neutrons. The mass number of sulfur is 32.
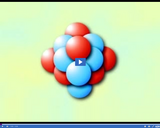
The mass number of an atom is the sum of its protons and neutrons.