The atmosphere is divided into different layers based on differences in temperature.
- Subject:
- Science
- Provider:
- Utah Education Network
- Author:
- Visual Learning Company
- Date Added:
- 02/28/2010

The atmosphere is divided into different layers based on differences in temperature.
The air closer to the Earth is denser than the air above it because the weight of atmospheric gases above compress it.

Satellites and space shuttles orbiting the Earth travel in the Exosphere.
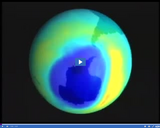
Pollutants can damage the ozone layer, creating large holes.

Particles of gas, called ions, become electrically charged in the ionosphere. The ionosphere plays an important role in transmitting radio waves.

The thermosphere is divided into two layers: ionosphere and exosphere.

Temperature plays an important role in delineating the layers of the atmosphere.

When the sun bombards Earth with large quantities of ions, they get trapped in the magnetosphere.
The ozone, located in the stratosphere, absorbs ultraviolet rays from the sun.

The stratopause separates the stratosphere from the mesosphere.
The stratosphere extends from the troposphere to 50 kilometers above Earth.

Located above the mesosphere, the thermosphere is a very hot layer of the atmosphere.
The tropopause separates the troposphere from the stratosphere.
The belt of charged particles surrounding the Earth, referred to as the Van Allen Radiation Belt, is located in the magnetosphere.
A Powerpoint guided lesson about atmospheres (April Mitchell)

In this feature, adapted from Interactive NOVA: "Earth," students explore the relationship between oxygen concentration and the well-being of various organisms by simulating a change in oxygen levels and observing what happens.

In this interactive activity from ChemThink, take a closer look at atomic structure, properties, and behaviors.
Explore the interactions between various combinations of two atoms. Turn on the force arrows to see either the total force acting on the atoms or the individual attractive and repulsive forces. Try the "Adjustable Attraction" atom to see how changing the parameters affects the interaction.
Explore the interactions between various combinations of two atoms. Turn on the force arrows to see either the total force acting on the atoms or the individual attractive and repulsive forces. Try the "Adjustable Attraction" atom to see how changing the parameters affects the interaction.

Students will use what they know about atoms & molecules to decide whether an example is an atom or a molecule.