
A carbon atom has 6 protons, 6 neutrons, and 6 electrons.
- Subject:
- Science
- Provider:
- Utah Education Network
- Author:
- Visual Learning Company
- Date Added:
- 02/28/2010

A carbon atom has 6 protons, 6 neutrons, and 6 electrons.

Atoms bond in ways that make their outermost energy levels complete with electrons, which stabilizes atoms.
A chemical compound is made up of two or more elements that are chemically combined or bonded. The combining of atoms to form new substances is called chemical bonding.
In covalent bonds, electrons are shared between two different atoms. Atoms are held together by the force of attraction between the positively charged nucleus and the shared electrons.
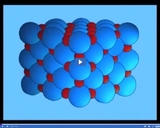
Many ionic compounds form a regular repeating pattern called a crystal lattice. Salt crystals have a crystal lattice that forms a cubic shape.

Niels Bohr discovered that electrons are arranged in energy levels. Each energy level can hold certain numbers of electrons. The first can hold 2, the second can hold up to 8, and the 3rd can hold up to 18.
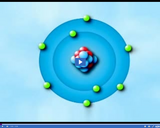
An atom is the smallest part of an element that still has all the properties of that element. Each different element is made up of a different kind of atom.
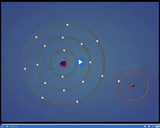
Hydrogen has a covalent bond with chlorine, which has seven electrons in its outermost energy level. When they bond, both outer energy levels become complete.

An ion is a charged atom that has gained or lost electrons.

In ionic bonding, oppositely charged ions are attracted to each other. In this animation, the sodium atom has one electron in its outermost level and the chlorine atom has seven electrons in its outermost layer.

In a metallic bond, positively charged ions are surrounded by a sea of electrons, which are attracted to multiple nuclei at the same time. Outer electrons tend to be very mobile.
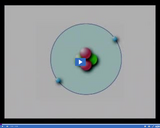
When an atom has an equal number of positively charge protons and negatively charged electrons, it has a neutral charge.
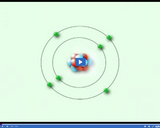
Atoms contain three types of subatomic particles: protons, which have a positive charge; neutrons, which have a neutral charge; and electrons, which have a negative charge.

Atoms contain three types of subatomic particles: protons, which have a positive charge; neutrons, which have a neutral charge; and electrons, which have a negative charge. Electrons orbit the nucleus at extremely high speeds.

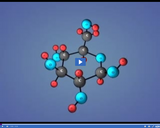
Water consists of a chemical compound formed between two hydrogen atoms and one oxygen atom. The atoms are connected to one another via a covalent bond.

Join an American Indian Interpreter as they share how the founding fathers looked to native governments when establishing their new republic.
In this optics activity, learners examine how polarized light can reveal stress patterns in clear plastic. Learners place a fork between two pieces of polarizing material and induce stress by squeezing the tines together. Learners will observe the colored stress pattern in the image of the plastic that is projected onto a screen using an overhead projector. Learners rotate one of the polarizing filters to explore which orientations give the most dramatic color effects. This activity can be related to bones, as bones develop stress patterns from the loads imposed upon them every day.
A ball and socket joint is made of a bone with a rounded end that fits inside a cup-like pocket of another bone, enabling bones to move in a circle. Hip joints and shoulder joints are examples.

Periosteum is a membrane that covers bones and plays a role in bone growth and repair. Beneath the periosteum is compact bone.

The skull protects the brain.