
Matter, such as this ice cream, is made up of many atoms.
- Subject:
- Science
- Provider:
- Utah Education Network
- Author:
- Visual Learning Company
- Date Added:
- 02/28/2010

Matter, such as this ice cream, is made up of many atoms.

Replicates the experiments J.J. Thomson conducted to develop his model of an atom. Light is produced when an electric current is passed through a tube. When a magnetic charge is introduced, the rays are deflected.
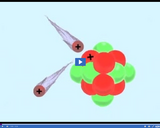
Like charges repel one another.

The mass number of an atom is the sum of its protons and neutrons. The mass number of sulfur is 32.
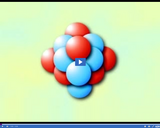
The mass number of an atom is the sum of its protons and neutrons.

The nucleus is the dense core of an atom. It contains over 99 percent of the mass of an atom, but occupies a miniscule amount of the space.
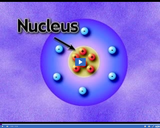
In Rutherford's model of an atom, positively charged particles are concentrated in a small, dense center of an atom called the nucleus. Negatively charged electrons are scattered around the nucleus.

Electrons are precisely one two-thousandth of the mass of a proton or neutron.

The nucleus of an atom contains positively charged protons and neutrally charged neutrons. Negatively charged electrons orbit the nucleus.

The food pyramid depicts the five major food groups: grains, fruit, vegetables, milk, meat and beans.
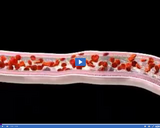
Arteries have thick walls so that they can handle the large force of blood being pumped through them by the heart. Blood in arteries is oxygen-rich.
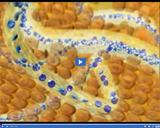
Blood in veins contains less oxygen than blood in arteries. It also contains waste gases, such as carbon dioxide.
The liquid part of blood is called plasma, which is made up of water and important substances, including vitamins, minerals, and nutrients.
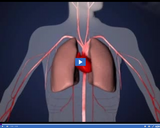
Oxygen mixes with blood in the lungs and is then pumped throughout the body.

Depicts millions of aluminum atoms. An atom is the smallest part of an element that still has all the properties of that element.

Lead and gold atoms differ from one another because they have different numbers of neutrons, protons, and electrons.

Atoms are the building blocks of matter.

Two hydrogen atoms form a simple covalent bond. Each atom has a single electron. When the atoms bond, they share electrons and fill their outermost energy level.

A carbon atom has 6 protons, 6 neutrons, and 6 electrons.

Atoms bond in ways that make their outermost energy levels complete with electrons, which stabilizes atoms.